FHIR Implementation Guide for ABDM
7.0.0 - active
FHIR Implementation Guide for ABDM
7.0.0 - active

FHIR Implementation Guide for ABDM - Local Development build (v7.0.0) built by the FHIR (HL7® FHIR® Standard) Build Tools. See the Directory of published versions
The International Patient Summary (IPS) is a standardized, minimal, and non-exhaustive set of clinical data intended to support continuity of care and patient safety across healthcare systems and borders. Defined by Health Level Seven (HL7) and International Organization for Standardization (ISO), it is a key artifact that enables safe, efficient exchange of health information, particularly during unplanned or cross-jurisdictional care.
A patient summary is not a complete health record but rather the essential minimum dataset required to ensure safe and effective care. By making critical health information readily available, patient summaries improve patient safety and support consistent, informed decision-making by clinicians across different sectors and domains.
An IPS bundle is an electronic health record extract containing essential healthcare information about the patient, including at least the mandatory elements defined in the IPS dataset. The dataset is:
As described in ISO 27269, the IPS was developed to support use cases such as unplanned, cross-border care.
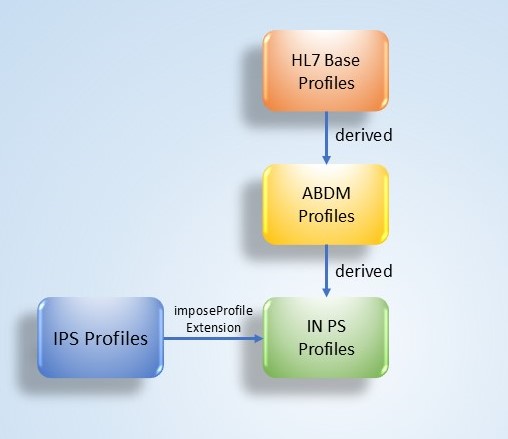
Indian Patient Summary (IN PS) is an implementable FHIR specification derived from the International Patient Summary (IPS) version 2.0.0, as defined by Health Level Seven (HL7) and the International Organization for Standardization (ISO). The IN PS aligns closely with the HL7 IPS-UV specification, incorporating localization for the Ayushman Bharat Digital Mission (ABDM). This approach ensures international interoperability while supporting national adoption and consistency across the Indian digital health ecosystem.
Indian Patient Summary (IN PS) follows the general principles and design conventions of the International Patient Summary Implementation Guide. Full details are available in the IPS IG.
Indian Patient Summary (IN PS) profiles are designed to ensure consistency with both national and international standards. All profiles defined in IN PS are derived from the Ayushman Bharat Digital Mission (ABDM) base profiles published for India, thereby aligning with the Ayushman Bharat Digital Mission (ABDM) ecosystem. At the same time, they are also made conformant to the International Patient Summary (IPS) profiles, ensuring global interoperability. To achieve this, the IN PS makes use of the imposeProfile extension, which allows implementers and validators to enforce dual conformance: the resource instance must be valid against both the Ayushman Bharat Digital Mission (ABDM) base profiles and the IPS profile. This ensures that while the national constraints are fully respected, the IPS constraints are not restated in the Indian Patient Summary profile but are still enforced at validation time.
The purpose of the Indian Patient Summary (IN PS) document is to define the logical structure and data set of the patient summary, encompassing documentation related IPS specification and patient-specific clinical information. It supports continuity of care and facilitates coordination across healthcare providers within and beyond national boundaries.
Indian Patient Summary (IN PS) is represented as a FHIR Bundle of type document. The Bundle contains multiple entries, with the first entry always being a Composition resource. All other entries are referenced from this Composition, ensuring a structured and comprehensive representation of the patient summary.
The IN PS document includes the following sections defined within the IN PS Composition, each serving a specific clinical purpose and ensuring standardized representation of patient information:
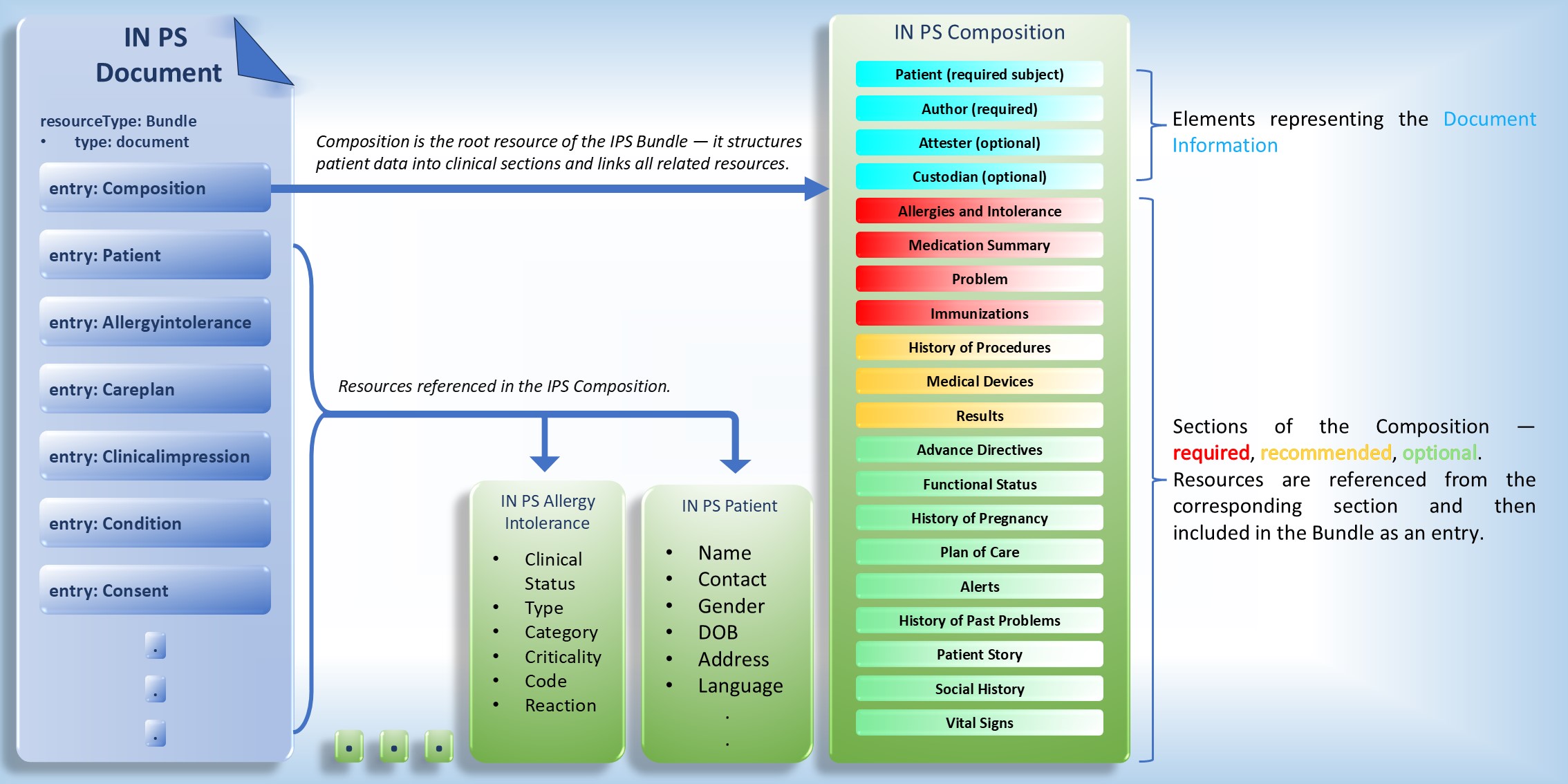
The IN PS Composition defines the overall structure and organization of the Indian Patient Summary (IN PS). It serves as the central resource that links all relevant clinical information — such as problems, medications, allergies, immunizations, procedures, and other sections — into a unified, standards-based patient summary document.
This Composition ensures a consistent and interoperable representation of patient data by defining metadata, section hierarchy, and references to underlying FHIR resources. It supports the accurate exchange of summarized health information across systems and care settings, facilitating continuity of care and clinical decision-making.
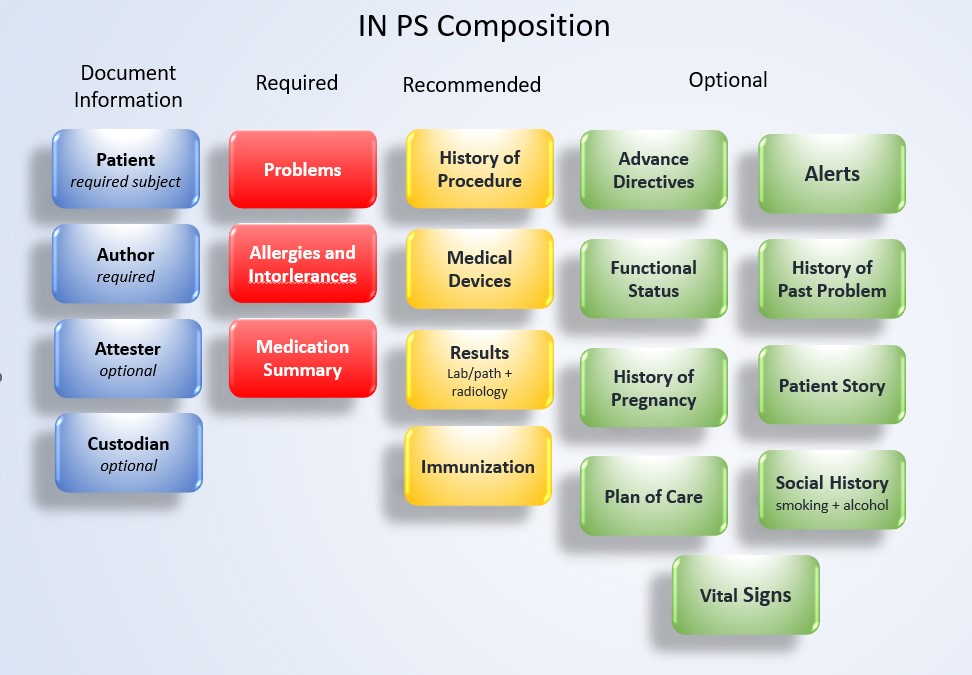
This part outlines the sections included in the IN PS Composition. Each section specifies its purpose, structure, and constraints for capturing clinical information within the patient summary.
The following sections are defined in the IN PS Composition.
This mandatory section provides a concise overview of the patient’s active or current health conditions that remain unresolved or under monitoring. It represents one of the core clinical components of a patient summary. Only active or current problems are to be listed, supporting continuity of care and providing a standardized overview of the patient’s health status.
Composition.section or a referenced resource at Composition.section.entry.This section reference the IN PS Condition profile, which defines constraints to represent active or current health problems or diagnoses relevant to the patient’s ongoing care. It is derived from the ABDM Condition profile and imposes additional requirements from the Condition (IPS) profile.
This mandatory section identifies known allergies and intolerance to support safe and effective treatment, helping clinicians avoid adverse events. It records active and relevant historical allergies or intolerance, along with associated reactions. The section describes the type of reaction (e.g., rash, anaphylaxis), the causative agent, and optionally, the severity and certainty. It ensures that critical allergy information is consistently communicated for patient safety.
Composition.section or a referenced resource at Composition.section.entry.The section reference the IN PS Allergies and Intolerances profile, which defines constraints on the AllergyIntolerance resource to represent active and historical allergies or intolerances relevant to the patient’s ongoing care. It is derived from the ABDM AllergyIntolerance profile and imposes additional requirements from the AllergyIntolerance (IPS) profile.
This mandatory section provides a list of medications relevant to the patient’s summary. It includes administered and non-administered medicines that are active or recently taken by the patient and are important for continuity of care.
Composition.section or a referenced resource at Composition.section.entry.
Only active or relevant medications should be included.This section reference the IN PS MedicationStatement or IN PS MedicationRequest profiles, ensuring accurate representation of the patient’s current medication profile and supporting safe and coordinated care.
- The IN PS MedicationStatement profile defines constraints on representing a patient’s medication usage. It is based on the ABDM MedicationStatement profile and imposes the additional requirements from MedicationStatement (IPS). It captures medications the patient is currently taking, has taken in the past, or is intends to take in the future, as reported by the patient, caregiver, or clinician.
- The IN PS MedicationRequest profile defines constraints for representing medication orders and requests. It is derived from the ABDM MedicationRequest profile and imposes additional requirements from MedicationRequest (IPS). It captures the intended medication, dosage, route, frequency, and timing.
This section provides information about the patient’s immunization status and relevant vaccination history. Its primary purpose is to enable accurate communication of a patient’s immunization record to support clinical care and continuity across organizations and borders.
Composition.section or through a referenced resource at Composition.section.entry.This section references the IN PS Immunization profile, which defines constraints for representing a patient’s immunization history. It is derived from the ABDM Immunization profile and imposes additional requirements from the Immunization (IPS) profile. The profile supports accurate recording of current and past vaccine administrations, ensuring consistent and standardized documentation across healthcare systems.
This section assembles relevant observation results obtained for the patient, including laboratory, pathology, and radiology findings. It provides essential diagnostic and monitoring information that supports clinical decision-making and continuity of care.
This section references the IN PS Observation Results - Laboratory/Pathology, IN PS Observation Results - Radiology, IN PS DiagnosticReport Laboratory/Pathology, and IN PS DiagnosticReport Radiology profiles for representing a patient’s diagnostic findings and individual observation results derived from laboratory, pathology, and radiology investigations. These profiles support both standalone observation reporting and grouped diagnostic reporting, enabling consistent representation of results across different clinical domains.
- The IN PS Observation Results - Laboratory/Pathology profile defines constraints for representing laboratory and pathology observation results. It is derived from the corresponding Observation profile and imposes additional requirements based on the Observation Results – Laboratory/Pathology (IPS) profile.
- The IN PS Observation Results - Radiology profile defines constraints for representing radiology observation results. It is derived from the ABDM Observation profile and imposes additional requirements based on the Observation Results – Radiology (IPS) profile, enabling standardized capture of imaging-related findings.
- The IN PS DiagnosticReport Laboratory/Pathology profile defines how collections of laboratory and pathology observations are grouped and summarized within a diagnostic report, providing clinical context, conclusions, and references to the underlying observations. It is based on the ABDM DiagnosticReportLab profile and imposes additional requirements from the DiagnosticReport (IPS) profile.
- The IN PS DiagnosticReport Radiology profile defines the structure for reporting radiology study results and interpretations, including narrative conclusions and references to associated imaging observations. It is derived from the ABDM DiagnosticReportImaging profile and imposes additional requirements from the DiagnosticReport (IPS) profile.
This section describes the patient’s past procedures that are relevant to the scope of the Patient Summary. It includes diagnostic, therapeutic, and surgical procedures that contribute to understanding the patient’s clinical history and prior interventions.
It supports a comprehensive clinical overview by documenting significant procedures such as invasive diagnostics (e.g., cardiac catheterization), therapeutic procedures (e.g., dialysis), and surgical interventions (e.g., appendectomy), providing important context for ongoing and future care decisions.
Composition.section or a referenced resource at Composition.section.entry.This section references the IN PS Procedure profile, which defines constraints on representing a patient’s past diagnostic, therapeutic, or surgical procedures relevant to the Patient Summary. It is derived from the ABDM Procedure profile and imposes additional requirements from the Procedure (IPS) profile.
This section documents the patient’s use of medical devices, whether implanted or external, that may influence current or future care. It provides essential information for clinicians to consider when planning treatment or interventions involving device-dependent patients.
It may include both narrative descriptions and coded entries detailing implanted devices (e.g., pacemakers, joint replacements) as well as external equipment (e.g., insulin pumps, oxygen delivery systems).
Composition.section or a referenced resource at Composition.section.entry.This section references the IN PS Device profile, which defines constraints on representing medical devices used by the patient, including implanted and external devices. It is derived from the ABDM Device profile and imposes additional requirements from the Device (IPS) profile.
This optional section captures information about a patient’s advance directives, outlining their preferences for care in situations where they may be unable to communicate or make decisions. It is particularly relevant for unplanned or emergency care scenarios.
The section may include narrative descriptions, references to supporting documents, and links to consent records that describe the patient’s expressed wishes for medical care. It supports ethical, informed, and patient-centered decision-making during critical situations.
This section references the Consent profile, which defines constraints for representing a patient’s advance directives and consent preferences. It supports consistent and structured documentation of patient choices related to medical care, enabling ethical and informed decision-making, particularly in unplanned or emergency care scenarios.
This optional section brings the most critical healthcare information to immediate attention. It highlights extreme or urgent problems that require heightened awareness from the attending clinician.
It may used to convey information specifically flagged to raise awareness of potential concerns, risks, or dangers to or from the patient, ensuring that critical issues are not overlooked during care delivery.
This section references the IN PS Flag Alert profile, which defines constraints for representing alert information relevant to the Patient Summary. It is derived from the ABDM Flag profile and imposes additional requirements from the Flag Alert (IPS) profile, enabling consistent representation of critical warnings and alerts that require special clinical attention and ensuring that critical risks or conditions are clearly communicated at the point of care.
This optional section describes the patient’s ability to perform activities of daily living and maintain functional independence. It captures information about physical, cognitive, or psychosocial limitations that may influence care planning, treatment decisions, and the need for ongoing assistance.
The section supports understanding of the patient’s overall well-being, particularly for individuals at higher risk or those requiring unplanned or long-term care. Future versions of this guide may include specific profiles for representing functional assessments and disability data.
This section references the IN PS Condition and ClinicalImpression profiles for representing functional limitations, disability-related conditions, and clinical assessments relevant to the patient’s functional status.
This optional section provides a record of the patient’s previous health problems or conditions that are no longer active. It offers historical context to support understanding of the patient’s current and future healthcare needs and contributes to continuity of care by documenting resolved or closed conditions relevant to the overall medical history.
This section references the IN PS Condition profile, which defines constraints for representing past or resolved health conditions. It is derived from the ABDM Condition profile and imposes additional requirements from the Condition (IPS) profile. It represents historical clinical conditions, supporting continuity and context in patient care.
This optional section presents the patient’s current pregnancy status and, when available, information about past pregnancies and their outcomes. It supports clinical assessment and care planning by providing relevant obstetric context.
The pregnancy status and history are represented using Observation resources, including an observation of the current pregnancy status and, optionally, a related observation of the estimated delivery date, as well as an observation summarizing pregnancy history when available.
This section references the IN PS Observation Pregnancy Status profile to represent the patient’s current pregnancy status, with an optional [IN PS Observation Pregnancy Expected Delivery Date] member to capture the estimated delivery date when applicable. It also references the IN PS Observation Pregnancy Outcome profile to summarize pregnancy history and outcomes. These profiles are derived from the corresponding ABDM ObservationWomenHealth profiles and impose additional requirements from the relevant IPS profiles Observation Pregnancy – Status (IPS), Observation Pregnancy – Expected Delivery Date (IPS), and Observation Pregnancy – Outcome (IPS) to ensure consistent and interoperable representation of pregnancy-related information within the patient summary.
This optional section contains narrative text, with optional supporting resources, that express what matters most to the patient. It reflects the patient’s perspective on their needs, strengths, values, concerns, and preferences to inform those providing care and support.
Information in this section may include the patient’s current sense of well-being and its date, the most important things they want others to know, dates of significant events or elements, matters that are personally important to them, and key people in their life.
Any FHIR resource type may be used to support or complement the narrative content where appropriate.
This optional section outlines the patient’s care plan, including goals, recommendations, and therapeutic actions intended to monitor, manage, or improve the patient’s condition. It provides a structured or narrative overview of planned care activities that support ongoing treatment and health management.
This section complements the overall clinical picture by summarizing therapeutic intentions and supporting coordination and continuity of care.
This section references the CarePlan profile, which defines constraints for representing planned care activities, goals, and therapeutic actions relevant to a patient’s ongoing management.
This optional section presents observations on social factors that may affect a patient’s health and well-being. These factors, such as tobacco or alcohol use, can serve as indicators of lifestyle and potential risk contributors.
This section references the IN PS Observation Social Alcohol Use and IN PS Observation Social Tobacco Use profiles.
- The IN PS Observation Social Alcohol Use profile defines constraints for representing a patient’s alcohol consumption. It is derived from the corresponding ABDM ObservationLifestyle profile and imposes additional requirements from the Observation Social History Alcohol Use (IPS) profile.
- The IN PS Observation Social Tobacco Use profile defines constraints for representing a patient’s tobacco use. It is derived from the corresponding ABDM ObservationLifestyle profile and imposes additional requirements from the Observation Social History Tobacco Use (IPS) profile.
This optional section compiles relevant observation results that record a patient’s vital signs, including primary indicators such as blood pressure, heart rate, body temperature, and respiratory rate.
This section references the ObservationResprate,ObservationOxygensat, ObservationHeartRate, ObservationBodyTemp, and ObservationBP profiles, which define constraints for representing a patient’s vital sign measurements—such as body temperature, blood pressure, heart rate, respiratory rate, and oxygen saturation—relevant to ongoing clinical assessment and monitoring.